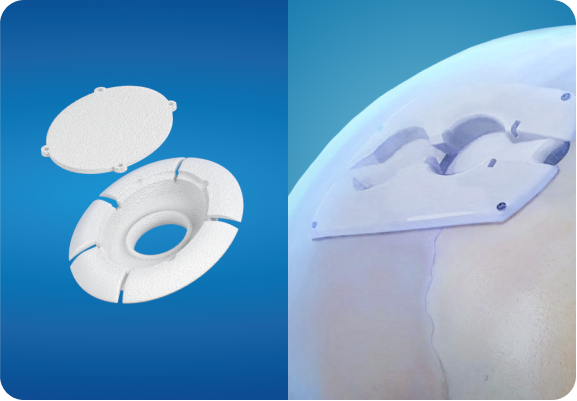
ClearFit® OTS
Longeviti ClearFit® OTS implants are available in a variety of shapes, sizes, and curvatures. ClearFit® off-the-shelf implants have the capability for bedside imaging.
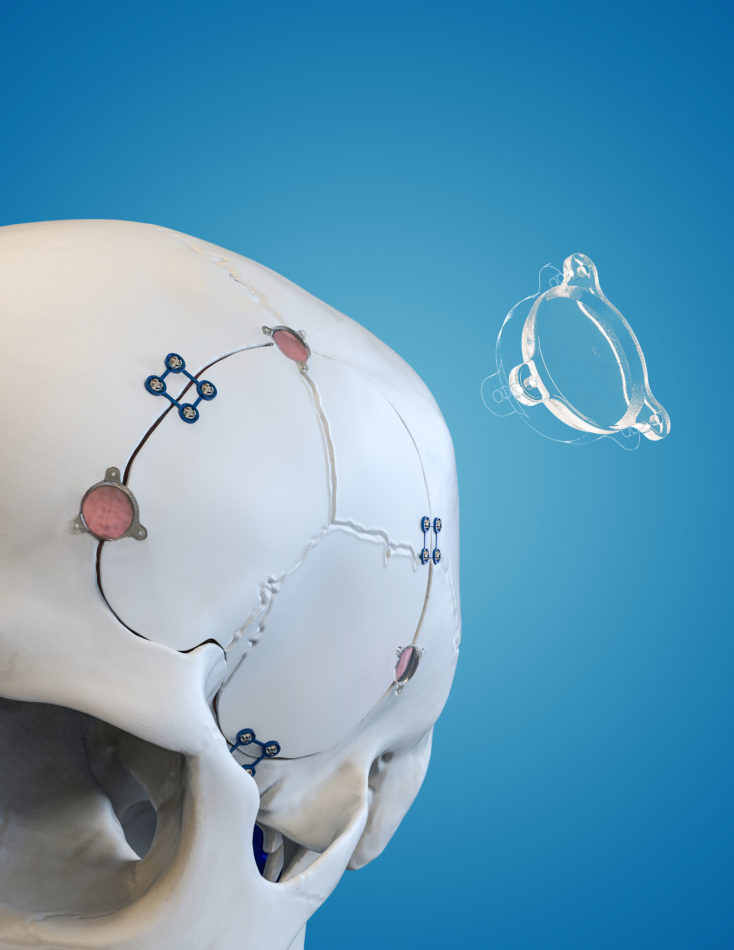
In 2016 Longeviti pioneered the development of the low-profile intracranial device platform – the world’s first customizable platform to address a growing unmet clinical need for patients undergoing complex brain surgeries.
Prior to Longeviti, there was no other solution that enabled the combination of technology within the cranium. Other solutions focus on only one of two primary neurological needs – either the functional requirements of the implant, or the reconstructive requirements. Longeviti has addressed both of these needs.
Since 2018, the company has brought several FDA-cleared implants to market. Longeviti’s technology is patent protected, view patents here.
Longeviti ClearFit® OTS implants are available in a variety of shapes, sizes, and curvatures. ClearFit® off-the-shelf implants have the capability for bedside imaging.

ClearFit® patient-specific implants are custom designed and tailored to an individual’s anatomy. These implants enable real-time ultrasound imaging through the skull, facilitating non-invasive monitoring of brain conditions post-surgery. This technology supports single-stage cranioplasty procedures, reducing the need for multiple surgeries and improving patient recovery times.

InvisiShunt® and InvisiLead are low-profile cranial implants designed to integrate seamlessly with the skull, restoring its natural contour while accommodating neurosurgical devices. Its design minimizes visible deformities and allows for the secure placement of functional implants, enhancing both aesthetic and medical outcomes.
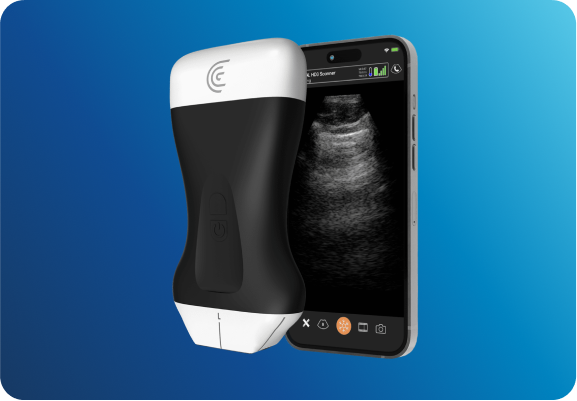
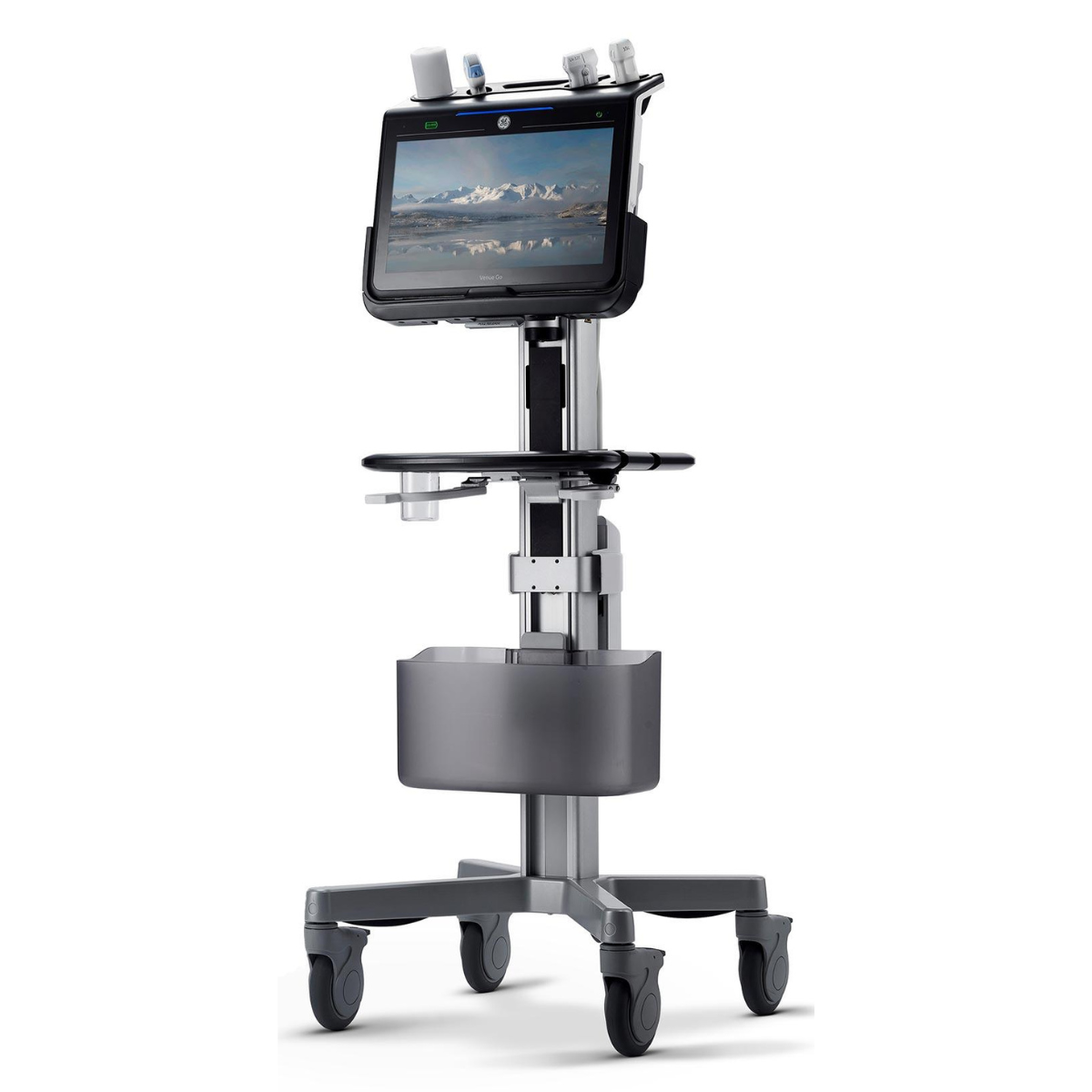
Longeviti works with Clarius Mobile Health and GE for high-resolution wireless ultrasound systems, providing images post-operatively through ClearFit® implants.